The following 65 Units are included in this category:
ID |
Unit (symbol) |
Definition |
Wikipedia page and other notes |
1 |
BTU [IT] (BTU_IT) |
1055.05585262 J |
https://en.wikipedia.org/wiki/British_thermal_unit#Definitions Can be considered an approximate definition The most widespread BTU uses the International Steam Table (IT) calorie, which was defined by the Fifth International Conference on the Properties of Steam (London, July 1956) to be exactly 4.1868 J. Therefore, the exact conversion factor for the International Table Btu is 1055.05585262 J, as shown in the calculation: OneLbmEqualsKg * 1000 * 5 / 9 * OneCalITEqualsJoules = 0.45359237 * 1000 * 5 / 9 * 4.1868 = 1055.05585262 |
2 |
BTU [Thermochemical TH] (BTU_TH) |
9489.1523804 / 9 J |
https://en.wikipedia.org/wiki/British_thermal_unit#Definitions Can be considered an approximate definition It is based on the definition of the thermochemical calorie, which is equal to 4.184 J. The calculation is similar to the one for the IT case: OneLbmEqualsKg * 1000 * 5 / 9 * OneCalTHEqualsJoules = 0.45359237 * 1000 * 5 / 9 * 4.184 = 9,489.1523804 / 9 ≈ 1054.35026448889 |
3 |
BTU [US, 59 °F (15 °C)] (BTU_US_59F) |
1,054.804 J |
https://en.wikipedia.org/wiki/British_thermal_unit#Definitions Can be considered an approximate definition 59 °F (15 °C) is the most widely used reference temperature for BTU definition in the US. Different values can be found in the literature. This value is the one most commonly used. See: https://en.wikipedia.org/wiki/British_thermal_unit (reporting 1054.80 J) https://physics.nist.gov/cuu/pdf/sp811.pdf (reporting 1054.80 J) |
4 |
calorie [IT] (cal_IT) |
4.1868 J |
https://en.wikipedia.org/wiki/Calorie#Definitions The Fifth International Conference on the Properties of Steam (London, July 1956) defined the International Table calorie as exactly 4.1868 J |
5 |
calorie [Thermochemical TH] (cal_TH) |
4.184 J |
https://en.wikipedia.org/wiki/Calorie#Definitions It is defined as 4.184 J exactly |
6 |
calorie [15 °C] (cal15) |
4.1855 J |
https://en.wikipedia.org/wiki/Calorie#Definitions Can be considered an approximate definition It is the amount of energy required to warm one gram of air-free water from 14.5 to 15.5 °C at standard atmospheric pressure. Experimental values of this calorie ranged from 4.1852 to 4.1858 J. The CIPM in 1950 (Comité international des poids et mesures (International Committee for Weights and Measures) 1950; PV, 1950, 22, 79–80) published a mean experimental value of 4.1855 J, noting an uncertainty of 0.0005 J. |
7 |
dyne centimetre (dyn·cm) |
10-7 J |
|
8 |
dyne metre (dyn·m) |
10-5 J |
|
9 |
dyne millimetre (dyn·mm) |
10-8 J |
|
10 |
erg (erg) |
10-7 J |
|
11 |
electronvolt (eV) |
1.602176634×10-19 J |
|
12 |
femtojoule (fJ) |
10-15 J |
|
13 |
foe (foe) |
1044 J |
|
14 |
gigaelectronvolt (GeV) |
109 eV |
https://en.wikipedia.org/wiki/Electronvolt Page referes to electronvolt |
15 |
gram-force centimetre (gf·cm) |
9.80665×10-5 J |
|
16 |
gram-force metre (gf·m) |
9.80665×10-3 J |
|
17 |
gram-force millimetre (gf·mm) |
9.80665×10-6 J |
|
18 |
gigajoule (GJ) |
109 J |
|
19 |
gigawatt hour (GW·h) |
3.6×1012 J |
https://en.wikipedia.org/wiki/Kilowatt-hour#Watt-hour_multiples |
20 |
joule (J) |
- |
https://en.wikipedia.org/wiki/Joule Equivalent to watt second, and newton metre |
21 |
kilocalorie [IT] (kcal_IT) |
4,186.8 J |
https://en.wikipedia.org/wiki/Calorie#Definitions Page referes to Calorie |
22 |
kilocalorie [Thermochemical TH] (kcal_TH) |
4,184 J |
https://en.wikipedia.org/wiki/Calorie#Definitions Page referes to Calorie |
23 |
kilocalorie [15 °C] (kcal15) |
4,185.5 J |
https://en.wikipedia.org/wiki/Calorie#Definitions Page refers to Calorie Can be considered an approximate definition, see cal15 above |
24 |
kiloelectronvolt (keV) |
103 eV |
https://en.wikipedia.org/wiki/Electronvolt Page referes to electronvolt |
25 |
kilogram-force centimetre (kgf·cm) |
9.80665×10-2 J |
The same as “kilopond centimetre” |
26 |
kilogram-force metre (kgf·m) |
9.80665 J |
The same as “kilopond metre” |
27 |
kilogram-force millimetre (kgf·mm) |
9.80665×10-3 J |
The same as “kilopond millimetre” |
28 |
kilojoule (kJ) |
103 J |
https://en.wikipedia.org/wiki/Joule#Multiples Equivalent to kilowatt second |
29 |
kilopond centimetre (kp·cm) |
9.80665×10-2 J |
The same as “kilogram-force centimetre” |
30 |
kilopond metre (kp·m) |
9.80665 J |
The same as “kilogram-force metre” |
31 |
kilopond millimetre (kp·mm) |
9.80665×10-3 J |
The same as “kilogram-force millimetre” |
32 |
kiloton of TNT (kt_TNT) |
1012 cal_TH |
https://en.wikipedia.org/wiki/Ton#Units_of_energy_and_power Based on that: 1 ton TNT ≈ 109 thermochemical calories Can be considered an approximate definition |
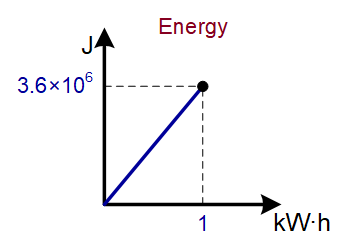
33 |
kilowatt hour (kW·h) |
3.6×106 J |
|
34 |
kilowatt second (kW·s) |
103 J |
https://en.wikipedia.org/wiki/Watt_second Page refers to watt second Equivalent to kilojoule |
35 |
pound-force foot (lbf·ft) |
12 lbf·in |
|
36 |
pound-force inch (lbf·in) |
0.1129848290276167 J |
|
37 |
millielectronvolt (meV) |
10-3 eV |
https://en.wikipedia.org/wiki/Electronvolt Page referes to electronvolt |
38 |
megaelectronvolt (MeV) |
106 eV |
https://en.wikipedia.org/wiki/Electronvolt Page referes to electronvolt |
39 |
millijoule (mJ) |
10-3 J |
|
40 |
megajoule (MJ) |
106 J |
|
41 |
megaton of TNT (Mt_TNT) |
1015 cal_TH |
https://en.wikipedia.org/wiki/Ton#Units_of_energy_and_power Based on that: 1 ton TNT ≈ 109 thermochemical calories Can be considered an approximate definition |
42 |
milliwatt hour (mW·h) |
3.6 J |
https://en.wikipedia.org/wiki/Kilowatt-hour#Watt-hour_multiples |
43 |
megawatt hour (MW·h) |
3.6×109 J |
https://en.wikipedia.org/wiki/Kilowatt-hour#Watt-hour_multiples |
44 |
newton centimetre (N·cm) |
10-2 J |
|
45 |
newton metre (N·m) |
1 J |
https://en.wikipedia.org/wiki/Newton_metre Equivalent to Joule and watt second |
46 |
newton millimetre (N·mm) |
10-3 J |
|
47 |
nanojoule (nJ) |
10-9 J |
|
48 |
ounce-force foot (ozf·ft) |
12 ozf·in |
|
49 |
ounce-force inch (ozf·in)
|
0.00706155181422604375 J |
|
50 |
poundal foot (pdl·ft) |
12 pdl·in |
|
51 |
poundal inch (pdl·in) |
0.0035116758411504 J |
|
52 |
picojoule (pJ) |
10-12 J |
|
53 |
petajoule (PJ) |
1015 J |
|
54 |
petawatt hour (PW·h) |
3.6×1018 J |
https://en.wikipedia.org/wiki/Kilowatt-hour#Watt-hour_multiples |
55 |
ton of TNT (t_TNT) |
109 cal_TH |
https://en.wikipedia.org/wiki/Ton#Units_of_energy_and_power Based on that: 1 ton TNT ≈ 109 thermochemical calories Can be considered an approximate definition |
56 |
teraelectronvolt (TeV) |
1012 eV |
https://en.wikipedia.org/wiki/Electronvolt Page referes to electronvolt |
57 |
therm [IT] (thm_IT) |
105 BTU [IT] |
|
58 |
therm [TH] (thm_TH) |
105 BTU [Thermochemical TH] |
|
59 |
therm [US, 59 °F (15.0 °C)] (thm_US_59F) |
105 BTU [US, 59 °F (15 °C)] |
|
60 |
terajoule (TJ) |
1012 J |
|
61 |
terawatt hour (TW·h) |
3.6×1015 J |
https://en.wikipedia.org/wiki/Kilowatt-hour#Watt-hour_multiples |
62 |
microjoule (µJ) |
10-6 J |
|
63 |
microwatt hour (μW·h) |
3.6×10-3 J |
https://en.wikipedia.org/wiki/Kilowatt-hour#Watt-hour_multiples |
64 |
watt hour (W·h) |
3600 J |
https://en.wikipedia.org/wiki/Kilowatt-hour#Watt-hour_multiples |
65 |
watt second (W·s) |
1 J |
https://en.wikipedia.org/wiki/Watt_second Equivalent to Joule and newton metre |
Most of the above definitions of the various units are EXACT with the exception of the cases that are highlighted in red color.
Note: In the above, a period (.) is used to indicate the decimal place and a comma (,) is used to separate groups of thousands.
Type of conversion relationship: DIRECTLY PROPORTIONAL (y=a∙x)
Example conversion diagram (see unit in red above):